INTRODUCTION
About 40% of stroke is attributed to atherosclerosis, which is affected by endothelial accumulation of cholesterol in the arterial wall [1]. Statin, an HMG-CoA reductase inhibitor used to lower blood cholesterol, has been proven effective in reducing incidence of stroke and myocardial infarction, thus being widely used in patients at risk for stroke [2,3]. However, as in other chronic illness, long-term compliance of statin use has been reported to be low in patients with dyslipidemia [4-6], and it is common that statins are discontinued arbitrarily or even against physicianŌĆÖs advice in routine clinical practice.
Although the causal relationship of discontinuation of statin and clinical events has not been confirmed, endothelial dysfunction can develop 24-36 hours after statin discontinuation [7,8] and is related to well-known risk factors for cardiovascular disease such as hypertension, obesity, and metabolic syndrome [9-11]. Discontinuation of statin increases the risk of cardiovascular disease in the long term and is associated with acute impairment of vascular elasticity regardless of cholesterol-lowering effects [8,12] and with poor prognosis and increased mortality in patients with cardiovascular disease [5,13,14].
Meanwhile, changes in vascular function associated with initiation or discontinuation of statin were assessed as vessel wall stiffness, mostly in arteries of the extremities or aorta [7,8]. However, assessing carotid artery stiffness may be clinically meaningful in terms of stroke risk prediction when considering the fact that the carotid artery is vulnerable to atheroma formation and stiffness increases faster with age in the carotid artery than in arteries of the extremities [15]. In a case-control study, a decrease in carotid arterial distensibility (CAD) was an independent predictor of ischemic stroke after adjusting for intimal medial thickness and major vascular risk factors [16]. Recent studies also showed associations between decreased arterial distensibility and less developed collateral circulation in patients with cerebral infarction due to large vessel occlusion [17] and hemorrhagic transformation in patients with ischemic stroke undergoing thrombolysis [18].
Therefore, in this study, we sought to examine whether there is a short-term change in CAD after discontinuation of statin among patients who preferred nonpharmacological therapy during statin use for dyslipidemia.
SUBJECTS AND METHODS
This study was performed on patients who preferred nonpharmacological therapy while taking statin due to dyslipidemia at the outpatient clinic. Patients included in the study met all of the following criteria: voluntary participation in the research, maintenance of statin at least 1 month before participation, drug compliance of 90% or more, decreased low-density lipoprotein (LDL)-cholesterol level by more than 30% after taking statin, and plasma cholesterol level of less than 200 mg/dL or LDL-cholesterol level less than 130 mg/dL at screening. Patients with the following conditions were excluded: history of cardiovascular or cerebrovascular disease, familial hyperlipidemia, significant liver or renal failure, chronic alcoholism or possibility of alcohol dependence, and physical inapplicability of carotid ultrasound. Patient recruitment and analysis were conducted under the approval of the Clinical Research Ethics Committee of Seoul Medical Center.
1. Measurement of CAD
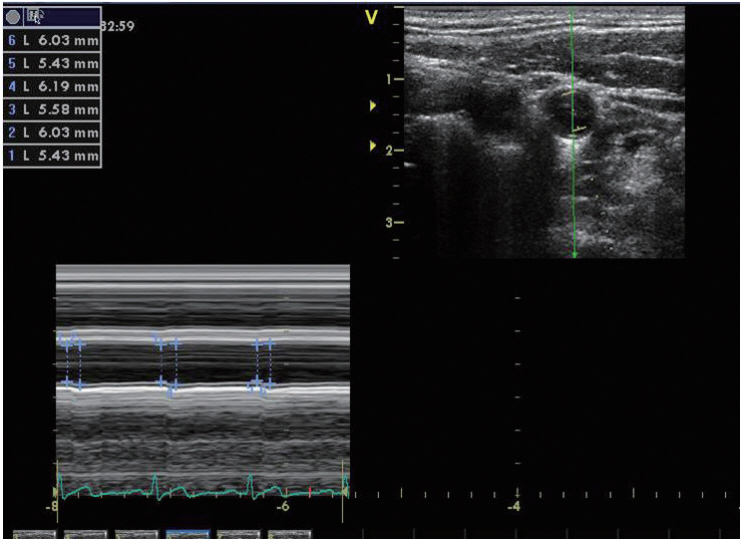
CAD was measured twice 7 days apart during statin use, and at 2, 7, and 30 days after discontinuation of statin (Fig. 1). All measurements were performed by one skilled technician, and a 7.5-12 MHz linear array transducer (Vivid 7, GE Healthcare, Milwaukee, WI, USA) was used. The measurement was conducted between 9 AM and 12 PM, and smoking and coffee and alcohol consumption were not permitted for 12 hours before the examination. The subjects were at rest for at least 5 minutes in the supine position and performed a 45┬░ head rotation in the opposite direction of the examined carotid artery. Measurements were made at 2 cm proximal to the origin of the bulb of the right common carotid artery. A digital video clip, when the near and par walls of the arterial cross-section were most visible in B-mode by varying insonation angles of the probe, was recorded during three cardiac cycles. Then, the recorded video clips were transferred to a separate computer and converted to M-mode images, and the mean maximum diameter of the vessel during systole and the mean minimum diameter during diastole were calculated during three cardiac cycles using a semi-automated edge-tracking approach (EchoPAC software version 6.2; GE Vingmed, Milwaukee, WI, USA) (Fig. 2). The vessel diameter was calculated by one researcher without knowledge of the information about the subject and timing of the measurement. During the examination, blood pressure was measured at 5-mintue intervals using a patient monitoring system (PAMO II, MEK, Seongnam, Korea) with the cuff placed on the right upper arm, and the mean of the blood pressures measured before and after the recording was used. CAD was determined by strain by dividing the difference between systolic and diastolic diameters by diastolic diameter and stiffness (╬▓) by adjusting strain by the effect of the systolic and diastolic blood pressures. Increase in ╬▓ or decrease in strain indicates increase in vascular stiffness, and the formula is as follows: [19]
1) ╬▓ = ln (systolic blood pressure / diastolic blood pressure) / [(diameter at systole ŌĆō diameter at diastole) / diameter at diastole]
2) Strain = (diameter at systole ŌĆō diameter at diastole) / diameter at diastole
2. Statistical analysis
Since the study had a case-crossover design to compare CAD measured before and after statin withdrawal, we used the Friedman test, a non-parametric repeated measure comparison method, when comparing values measured during or after statin use. The corresponding comparison between the first measured value and the measured value at each time point was conducted using the Wilcoxon signed-rank test. Comparison of the mean between the independent groups was conducted using the Mann-Whitney U test. A two-sided p-value <0.05 was used as the criterion for statistical significance. All statistical analyses were performed using SPSS for Windows version 20 (SPSS Inc., Chicago, IL, USA).
RESULTS
Among the 37 patients who agreed to participate in the study, 21 patients who completed all the examinations were analyzed. The mean age of the subjects was 60.8┬▒10.2 years, and seven patients were male. The prevalence of hypertension and diabetes was 47.6% and 19%, respectively. The most commonly used statin was rosuvastatin 10 mg (10 patients), and other statins were atorvastatin 10 mg (seven patients), rosuvastatin 5 mg (three patients), and pitavastatin 2 mg (one patient). The mean LDL-cholesterol concentration during statin use was 82.5┬▒19.7 mg/dL, while the mean value was 150.1┬▒19.5 mg/dL after 30 days of discontinuation.
At the first examination, the mean diameters of the common carotid arteries during systole and diastole were 6.4┬▒0.8 mm and 5.9┬▒0.8 mm, respectively. The mean difference between systolic and diastolic diameter was 0.47 mm. The mean diameter in systole and mean difference between systolic and diastolic diameters were significantly different between the measurements, which were smallest when measured at 2 days after discontinuation (Table 1).
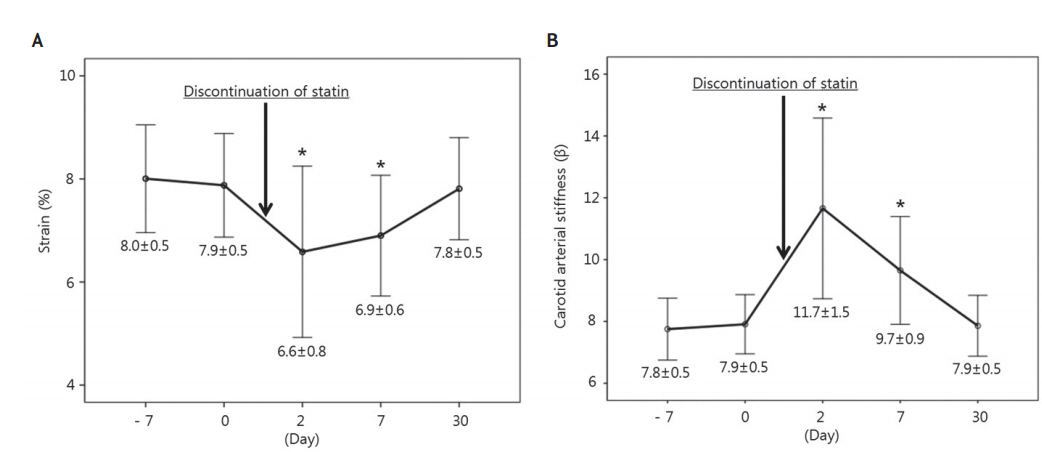
The mean strain was significantly different between the measurements (Friedman test, p=0.007; Fig. 3A), which was lowest (6.6┬▒0.8%) at 2 days after discontinuation but highest (8.0┬▒0.5%) on the first examination during statin use. Compared with the strain on the first examination during statin use, the strain measured at 7 days after discontinuation was significantly lower (p=0.002), while that measured at 30 days was not significantly different (p=0.715).
In contrast with the strain, the mean ╬▓ was highest (11.7┬▒1.5) 2 days after discontinuation, which was significantly different between the measurements (Friedman test, p<0.001). Likewise, compared with the ╬▓ on the first examination during statin use, ╬▓ measured at 2 and 7 days after discontinuation was significantly higher but that measured at 30 days was not significantly different (Fig. 3B).
To determine if there is any different effect of statin dose on CAD, subjects were divided into rosuvastatin 10 mg group (n=10) and the remaining statins group (rosuvastatin 5 mg, atorvastatin 10 mg, pitavastatin 2 mg; n=11) according to equivalent dose. The changes in LDL-cholesterol concentration after discontinuation were 78.6┬▒22.0 mg in the rosuvastatin 10 mg group and 57.6┬▒19.6 mg in the remaining statins group but not significantly different between groups (p=0.132, Mann-Whitney U test). There was also no significant difference between groups in terms of changes in the mean strain (Friedman test, p=0.654) and ╬▓ (p=0.705) measured before and 2 days after statin discontinuation.
DISCUSSION
In this study, we found that CAD in patients receiving statin without a history of cerebrovascular disease increased shortly after statin discontinuation. This is consistent with previous findings of reduced vascular elasticity after statin withdrawal observed in arteries other than the carotid artery, such as the brachial artery [7,20,21].
Although it is not known whether cardiovascular diseases, including stroke, increases shortly after statin withdrawal, the pleiotrophic effect of statin occurs rapidly at the beginning of treatment. In a study in which patients without history of stroke were treated with daily atorvastatin 80 mg, changes in CAD were observed, which was increased by 13% at 30 days of administration [22]. In contrast, abrupt withdrawal of statin may result in decreased nitric oxide (NO) synthesis of the vascular endothelial cell, which is related to vascular dysfunction [8,12]. Clinically, statin withdrawal increased the risk of 1-year mortality in patients with ischemic stroke [5], and patients who discontinued statin on the first 3 days of stroke were reported to have a poor prognosis and greater volume of cerebral infarction at 3 months than those who did not [14].
Statin not only reduces the production of reactive oxygen species of vascular cells in addition to lipid-lowering effect but also activates endothelial nitric oxide synthase (eNos), which produces the important vasodilator NO [23]. In the experimental animal study, the expression of eNOS and production of NO after statin withdrawal decreased on the 2nd day of discontinuation. This suggests that Rho protein, which inhibits eNOS, accumulates in the cytoplasm as an inactive form during statin therapy but is overexpressed and inhibits eNOS after statin withdrawal [24].
Therefore, our finding that carotid artery stiffness increased transiently on the 2nd and 7th day after statin discontinuation seems to result from decreased NO production, associated with eNOS suppression by excessive transition of Rho proteins into the cell membrane. Recovery of CAD at 30 days after statin withdrawal was supposed to result from depletion of accumulated Rho proteins. It is known that the decrease in endothelial NO accentuates atherosclerosis by decreasing vasodilatory capacity of vascular endothelium, aggregating platelets, and promoting binding of inflammatory cells to the vessel wall [25].
Statins for the primary prevention of cardiovascular disease is recommended to be used depending on target LDL-cholesterol concentration in patients with a 10-year risk of cardiovascular disease greater than 7.5% [26]. The target LDL-cholesterol concentration is suggested to be less than 100 mg/dL when statins are used for secondary prevention in patients with a history of stroke [27]. However, because there is no guideline on the duration of statin treatment, a temporary disruption may occur when the target blood lipid concentration is achieved and a patient prefers to switch to nonpharmacological therapy. Furthermore, compliance to medications such as statin has been reported low in chronic illness. In a study conducted in patients with coronary artery disease, less than half of patients maintained lipid-lowering agents for 6 months to 1 year after discharge [28]. Similarly, in a study on the elderly population, less than 50% maintained statin for more than 6 months [4].
In clinical practice, it is common to see patients without history of cardiovascular disease often discontinue statin themselves if the target LDL-cholesterol concentration is achieved during long-term medication. However, considering the short-term decrease in CAD after statin withdrawal observed in this study, precaution and attention should be paid to the risk of arbitrary statin discontinuation although its association with clinical events is not yet known. Particularly, further studies in patients having stroke or other cardiovascular diseases during statin use are needed to elucidate whether the decrease in vascular elasticity caused by unintentional statin discontinuation during the acute phase of treatment affects the disease prognosis.
This study has some limitations. Since only outpatients with dyslipidemia and without history of cerebrovascular disease who preferred to discontinue statin use were eligible to this study, the number of patients enrolled might not be enough to determine the differences in the changes of vascular elasticity according to the change in LDL-cholesterol concentration in the analysis considering the equivalent statin dose. It is also a weak point that the use of antihypertensive agents that can affect the elasticity of the blood vessels and effect of blood pressure on distensibility were not analyzed because of the small sample size. However, generalizability may be a strong point of this study since the subjects were enrolled from the circumstances of real practice.
Finally, it should be revealed through further researches whether the effect of statin withdrawal on blood vessel elasticity differs by types or doses of statin and whether the risk of cardiovascular disease actually increases in the short term after statin withdrawal.