INTRODUCTION
Carpal tunnel syndrome (CTS) is an entrapment neuropathy affecting the median nerve with a life-time prevalence of about 8% in the adult population.1 Nerve hydrodissection (HD) is a widely used technique that involves the injection of fluid to separate the nerve from the surrounding structures.2
This review is aiming to discuss the detailed approaches of ultrasound (US)-guided HD, evaluate their safety and efficacy, and answer the unanswered questions as regards the best approach, several injectates, and related outcomes with evidence-based clinical recommendations. Several systematic reviews, meta-analysis, pilot, and randomized-controlled trials were discussed and compared for each point of discussion.
POSSIBLE MECHANISMS
A potential benefit of HD is to release the pressure on the “free nerves supplying the main nerves,” called “nervi nervorum.”3 Early, the compression of the vasa nervorum would affect venous outflow, causing the accumulation of toxins in the affected part. Then, the lymphatic drainage, located outside the epineurium, would undergo compressive effects.2 HD can also decrease the gliding resistance of the median nerve within the carpal tunnel, which is considered a principal patho-mechanical cause of nerve injury.4
1. Efficacy
Although the results of the studies were dispersed, several studies have confirmed the efficacy of HD in CTS. A study by Wang et al.5 included 64 patients divided into interventional and control groups. Both groups experienced improvements in functional and electrophysiological scores. However, there were no upcoming significant differences.5 Another pilot study by Schrier et al.6, which reached the same results with the hydrodissected group, did not show additional improvement.
On the other side, in a study by Goru et al.7, 70% of patients reported significant improvement, 8% had temporary improvement with recurrence, and 28% failed to improve. They recommended the use of ultrasound-guided HD for the treatment of mild to moderate CTS before offering surgical treatment, especially for patients with significant medical problems or refusing surgery. US reduces injection-related complications, improves patient satisfaction, and improves patient compliance.7
Many other studies had then proved the efficacy and safety of HD with superior benefits than traditional methods.8,9 In a systematic review by Yang et al.10, they found that US-guided injection yielded favorable results for symptom severity and functional status. They recommended US-guided HD as a treatment for patients with CTS.10
3. Long-term outcome
HD is now considered a novel approach for the treatment of CTS with safe and outstanding long-term effects. A retrospective study by Li et al.12 investigated the long-term outcome; 88.6% of patients reported an effective outcome, and 11.4% had a poor outcome after a mean of 2.2 injections with a mean of 1–3 years’ post-injection follow-up. This was significantly related to the severity level. Patients with mild, moderate, and severe grades, respectively, required an average of 1.7, 2.4, and 2.6 injections to reach an effective outcome.12
HD METHODS
1. In-plane approach
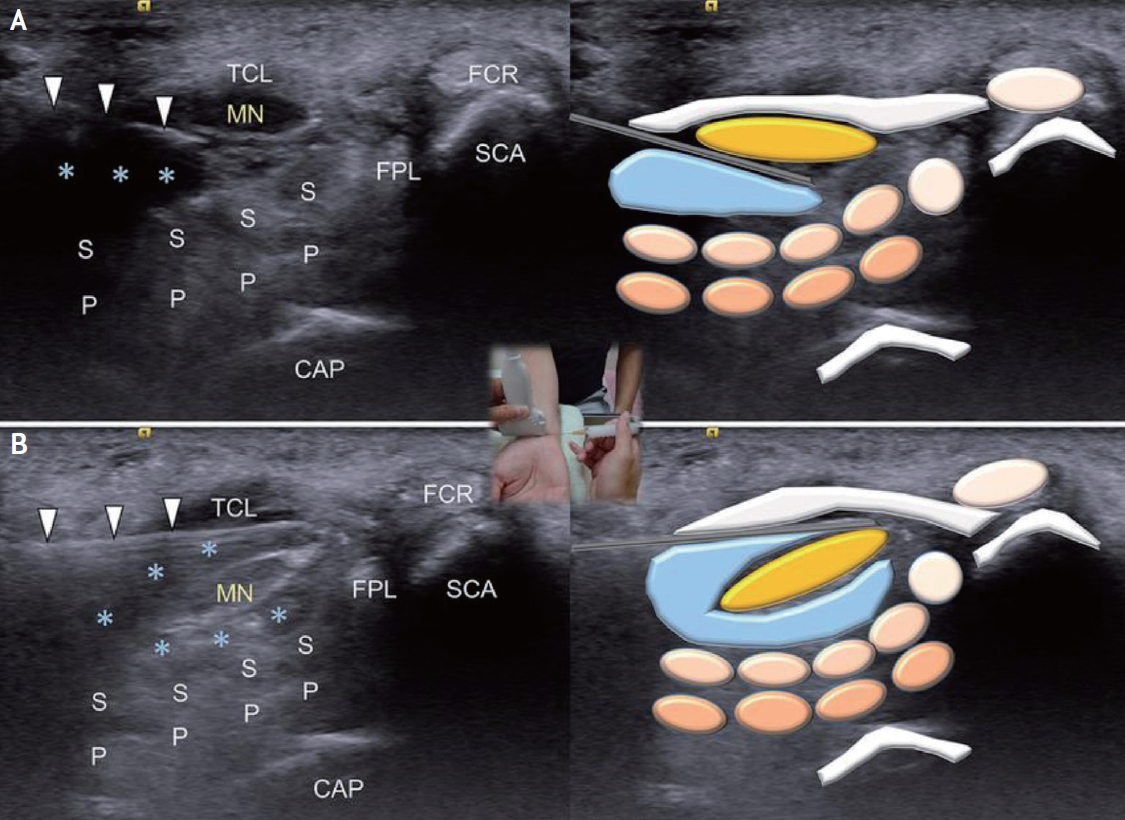
The needle is in-plane with the transducer perpendicularly to the long axis of the nerve, and the tissues above and below the nerve are hydrodissected with the bevel of the needle directed downwards for the superficial part of the nerve to avoid nerve injury, then shifted upwards for the inferior part of the nerve. The hydrodissected nerve appears oval and is surrounded by anechoic fluid on the US after injection (Fig. 1).13
In-plane approach could be performed from either the ulnar or radial sides, with limited studies comparing both sides. A study by Babaei-Ghazani et al.14 found that the radial approach could be at least as effective as the more common ulnar approach with longer pain relief.
2. Out-of-plane approach
The needle is parallel to the long axis of the nerve, and the probe is first perpendicular and then parallel to the long axis of the nerve. This approach facilitates hydrodissection of longer segments of the nerve.13
3. In-plane vs. out-of-plane approach, which one is preferred?
Lee et al. 15 found that US-guided injection using an inplane ulnar approach might be more effective than outplane or blind injection as regards the degree of symptom improvement and the change in electrophysiological and ultrasonographic findings.
ULTRASOUND-GUIDED VS. BLIND INJECTIONS IN CTS
In Chen et al.16 study, they found that US-guided injection may be more effective with an earlier and better improvement of symptom relief, greater improvements in the Semmes-Weinstein monofilament test, and sensory nerve conduction velocity.
A systematic review suggested that US-guided injection was more effective than blind injection in symptom severity improvement; however, there were no significant differences in functional status or electrodiagnostic improvements between the 2 methods.17
1. Safety of US-guided HD of nerves
A potential nerve injury is expected whenever a doctor injects around the nerve. Jeng et al.18 suggested that nerve damage is rare due to the polyfascicular architecture of the peripheral nerve and nerve fiber dispersal within the nerve.
2. Different injectates; which is the best one?
1) HD with normal saline (NS) in CTS
Traditionally, a large volume of NS, a small volume of steroid, and a local anesthetic solution are used for HD of the nerves.19,20 A clinical trial by Wu et al.21 for patients with mild-to-moderate CTS compared the effect of HD with 5 mL NS to subcutaneous injection of 5 mL NS above the carpal tunnel, with confirmed efficacy of HD probably via detaching the entrapped nerve and promoting blood flow while decreasing nerve compression injury. However, the minimal required amount of injectate for the HD effect is still unknown.21
2) HD with dextrose 5% in water (D5W) in CTS
D5W iso-osmolarity would cause less irritation and be harmless for the nerve.22 The exact therapeutic mechanism of D5W in treating CTS remains unclear and may be related to inhibiting transient receptor potential vanilloid receptor-1 in blocking the release of substance P and calcitonin gene-related peptide.23 Chronic neuropathic pain may signify glycopenia around the corresponding nerves. Injecting dextrose may correct this glycopenia and consequently reduce neuropathic pain.14
3) HD with hyaluronic acid (HA) in CTS
The possible mechanisms of HA on CTS may be the antiadhesion, anti-inflammatory, and nerve regeneration effects through which HA can dissect the median nerve MN from the surrounding connective tissue and improve nerve mobility.24
4) Platelet-rich plasma (PRP)
PRP, also known as autologous concentrated platelets, contains numerous bioactive factors, including transforming growth factor-β, platelet-derived growth factor, and vascular endothelial growth factor, which could promote tissue repair and regeneration. When compared to traditional therapy, PRP was shown to have better efficacy for 3 to 6 months in patients with mild-to-moderate CTS compared with splints or corticosteroid injections, respectively.25,26
5) HD with steroids
Corticosteroid injection is an extensively used and accepted treatment for CTS. However, there is no guideline as to which corticosteroid should be used as the standard treatment. Triamcinolone acetonide is a commonly used particulate steroid that can cause permanent nerve injury if it is accidentally injected into the nerve.27 Conversely, dexamethasone sodium phosphate is a nonparticulate steroid that would not cause permanent nerve injury and could be considered a more effective alternative in the treatment of CTS patients.28
OTHER INJECTANTS
1. Ozone injection for CTS treatment
Ozone is now considered a promising agent for many musculoskeletal disorders. These therapeutic effects are obtained by ameliorating tissue oxygenation, accelerating glucose usage in cellular metabolism, improving protein metabolism, increasing erythrocyte activity, inhibiting inflammatory mediators, and decreasing joint oxidative stress.29
In a randomized controlled trial, 40 patients with mild or moderate CTS were included, with outcomes reassessed 10 weeks after the treatment. The functional scales and visual analog scale (VAS) improvements were more remarkable in the ozone group than in the control group.30
2. Does the volume of injectant matter?
This was a debatable issue with dispersed results. In a study by Wang et al.27, subjects were randomly assigned to either US-guided HD with a mixture of 1 mL of triamcinolone acetonide (10 mg/mL), 1 mL of 2% lidocaine, and 8 mL of normal saline versus US-guided injection of 1 mL of triamcinolone acetonide (40 mg/mL) and 1 mL of 2% lidocaine. They found that both groups experienced improvement in functional and electrophysiological outcomes with no significant difference.27
Another pilot study by Schrier et al.6 involved 20 CTS patients randomized into an US-guided HD with a 5 mL betamethasone injection versus a 2 mL injection. They found that symptomatic and functional scores improved for both groups, and the hydrodissected group did not show additional improvement. However, a smaller sample size was a strong limitation.6
Nevertheless, in a randomized, double-blinded, three-arm trial, Lin et al.31 found that US-guided injection of 4 mL of 5% dextrose provided better efficacy than with 1 and 2 mL based on symptom relief and functional improvement at the 1st, 4th, and 12th weeks post-injection. There was no significant difference between the three groups at the 24-week post-injection follow-up.31
They also further investigated the effect of different injectate volumes on US parameters and the correlation to clinical outcomes in a post hoc analysis. The cross-sectional area (CSA), which is a primary sonographic parameter used to assess the median nerve pathology, decreased significantly at all follow-up time points in the 2 mL group (p=0.005) and the 4 mL group (p=0.015). The mean change in mobility from baseline showed a greater improvement in the 4 mL group than in the other groups at the 1st week post-injection. For clinical outcomes, a negative correlation between the VAS and mobility at the 1st (p=0.046) and 4th weeks (p=0.031) post-injection in the 4 mL group was observed. They concluded, “HD with higher volume yielded better nerve mobility and decreased CSA of the median nerve, but no changes in nerve elasticity”.32
3. Comparison of different injectates for CTS (Table 1)
2) D5W vs. steroids+NS
Another study by Wu et al.34 compared a median single-session HD in two groups of patients. Patients were injected with 5 mL of D5W (dextrose group) vs. 3 mL of triamcinolone acetonide mixed with 2 mL of normal saline (steroid group), with a significant difference for the dextrose group as regards functional and clinical improvement.34
3) HA vs. NS
In the study of Elawamy et al.35, patients were allocated equally into either group 1 (HD with HA+10 mL NS injection) or group 2 (HD with 10 mL NS only). They found that HD with HA is more efficient, offering a rapid onset of pain relief and functional improvements and better MN conduction in patients with CTS over a 6-month follow-up duration.35
4) HA vs. steroids
A study of Alsaeid36 was carried out to compare the efficacy of HA versus dexamethasone in US-guided HD of the MN in mild to moderate cases of CTS. HA significantly improved patients compared to the steroid group as regards the Boston Carpal Tunnel Questionnaire (BCTQ), electrophysiological studies (SNCV, DML), and sonographic data at 1 week, 1 month, 3 months, and 6 months follow-up post-injection times.36
5) Ozone (O2-O3) injection vs. corticosteroid injection
In a comparative study by Babaei-Ghazani et al.37, both groups showed improvement in BCTQ and VAS at week 6, and this improvement continued until the 12th week after the injections. However, electrodiagnostic values of sensory nerve action potentials and compound motor action potentials latency and ultrasound CTS criteria showed significant improvement only among the subjects in the corticosteroid group at 6 and 12 weeks after the injection (p<0.05).37
4. Different injectates; which one is preferred?
A comparative study of the commonly used injectates was done for patients with severe CTS for more than 6 months based on CSA >15 mm2; with failed conservative treatments, they were allowed to choose from NS, D5W, PRP, and HA. They found that single doses of PRP, D5W, and HA were more efficient than NS as regards symptomatic and functional improvement, as well as decreased CSA in MN. Single injections of PRP and D5W seemed more effective than those of HA within 6 months post-injection, although PRP was slightly better than D5W.38
For reducing CSA, PRP, and HA seemed more effective than D5W. HA was the most effective at the 1st month post-injection (HA>PRP>D5W), and PRP was the most effective at the 6th month (PRP> HA>D5W).39
DISCUSSION AND RECOMMENDATIONS
Hydrodissection HD of the entrapped median nerve is considered a safe and efficacious approach for treatment of mild to moderate CTS and could be offered to patients with severe CTS if surgery is refused or can’t be done due to other medical comorbidities. The use of US-guided HD could improve symptom severity, functional status, and the US parameters of nerve entrapment. HD has a long-term efficacy outcome; 88.6% of patients reported an effective outcome, and this may vary according to the initial severity status.12
There are two primary methods of US-guided HD of peripheral nerves: the in-plane and out-of-plane approaches. The radial in-plane approach is much preferred than the commonly used ulnar approach as it has a more upcoming pain relief effect.14
In general, the in-plane approach is much preferred over the out-of-plane or blind approach. An out-of-plane approach is recommended only if a larger section of the nerve should be hydrodissected.13,15
Different types of injectants are available and studied, with no clear indication of use for each type. However, PRP, D5W, and HA were more efficient than NS as regards subjective and objective improvement. PRP and HA were more effective for long-term nerve recovery, while HA was preferred for the earliest response. So, we recommend making the choice based on every patient’s severity scale and electrophysiological scales which means that for patients with mild form and severe pain, we suggest using HA, while PRP is suggested for those with moderate to severe form to enhance the nerve repair mechanism.33-35,39 Dexamethasone injection is more safe than the commonly used triamcinolone.28
Injection of large volume could be a better option in order to dissect a larger area, provide a better environment for the nerve repair mechanisms and release the vascular and neural compressive elements.32
HD should be performed by an expert sonographer with repeated training programs.
More larger size and meta-analysis studies are still needed to get the final recommendations and guidelines of use of HD for carpel tunnel syndrome.