Muscle Ultrasound in Clinical Neurology: Diagnostic Uses and Guidance of Botulinum Toxin Injection
Article information
Abstract
Muscle ultrasound (MUS) is increasingly used by neurologists, neuropediatricians, neurosurgeons, specialized radiologists and anaesthesiologists for the imaging-supported diagnosis of neuromuscular disorders. Especially, MUS is highly sensitive in detecting fasciculations in motor neuron diseases, and in revealing intensive care unit acquired weakness. Hereditary and inflammatory myopathies are associated with distinct patters of echo-intensity changes of affected muscles. Moreover, MUS can be used for guiding needle biopsy of muscle lesions, and for targeting intramuscular botulinum neurotoxin (BoNT) injection in neurological disorders with muscle hyperactivity. MUS-guidance of BoNT injection is especially recommendable in complex cervical dystonia, in task-related hand dystonia (writer’s cramp, musician dystonia), and in children and adolescents with cerebral palsy. Modern ultrasound technologies such as sono-elastography, tissue Doppler, and high-definition microvasculature imaging allow for novel diagnostic and therapeutic uses. Recently, an international expert group reported consensus guidelines for neuromuscular ultrasound training. The present review provides a concise overview of well-established diagnostic and therapeutic applications of MUS in clinical neurology, with specific focus at MUS for targeting intramuscular BoNT injections.
INTRODUCTION
Muscle ultrasound (MUS), also referred to as myosonography, has been applied in the field of neurology since the early 1980s, even though ultrasound (US) image resolution was low at that time.1-4 With the advance of imaging technology MUS became more feasible, and meanwhile numerous diagnostic and therapeutic applications have been developed.5-8 In 2019, an international expert group reported consensus guidelines for neuromuscular US training including both, assessment of nerves and muscles.9 According to these guidelines the following procedural skills are required specifically for MUS: basic level—(1) differentiating bone, muscle, nerve, vasculature, and tendons; and (2) discrimination of normal vs. abnormal muscle (e.g., Heckmatt scale, atrophy, echogenicity, and thickness measurement); intermediate level—(1) patterns of muscle involvement in generalized neuropathy/myopathy; and (2) dynamic muscle ultrasound (e.g., fasciculations/diaphragm excursion); advanced level—(1) quantifying muscle echogenicity; (2) needle guidance for intramuscular injections (e.g., botulinum toxin); (3) needle guidance for muscles with increased risk (e.g., diaphragm); and (4) advanced MUS techniques, such as elastography, 3D, extended field of view, and contrast agents. The present review provides an overview of well-established applications MUS in clinical neurology, with specific focus at MUS for targeting intramuscular botulinum neurotoxin (BoNT) injections. This article follows the outline of the author’s lecture held on November 4, 2022, during the second day of the Joint Conference of the Korean Society of Neurosonology (KSN) and the Neurosonology Research Group of the World Federation of Neurology (NSRG-WFN) in Seoul, Korea.
TECHNICAL CONSIDERATIONS
US machines used for MUS are usually identical to those used for vascular US. In principle, all contemporary US machines can be used if equipped with an appropriate linear-array or, alternatively for deep muscles, a curved-array transducer. The applied US frequency determines both, the image resolution and the penetration depth. Increasing frequencies increase image resolution, but reduce penetration depth and vice versa. Profound and large target muscles in the neck, trunk and leg are best visualised with US frequencies of about 7.5 (5–10) MHz.10 The assessment or targeting of superficial and small muscles in the face or arm region requires optimal resolution and therefore US frequencies in the high-frequency range of about 18.0 (15–25) MHz (Table 1).6,11,12 For standard applications of B-mode MUS, the US system settings are adapted at high dynamic range and at medium-to-high persistence. Contemporary US systems are usually delivered by the manufacturer with a choice of ‘musculoskeletal’ presets that can be selected for MUS.6 For optimal image quality a rectangular probe position with only moderate pressure on the underlying tissue is recommended. Angular deviation of the probe position may reduce tissue echogenicity, increased pressure of the probe towards the skin may increase tissue echogenicity. Rectangular probe position in relation to bone surfaces is recognized by the very bright appearance of the surface of the referring bone. To allow for improved comparability it has been recommended that the left side of the US screen shows the medial, ulnar, cranial or proximal side of the examined body part and the right side the lateral, radial, caudal and distal side.13 Alternatively, at axial transection of a body part the left side of the US image can display the left side of the referring body region as seen from the investigator which may be more intuitive especially if MUS is applied for injection guidance.6
ULTRASONOGRAPHY OF NORMAL MUSCLE
Normal muscles can be identified easily on US by their “starry night” appearance on transverse view and pennate appearance on longitudinal view, the identification of their origin and insertion and their diameter changes on contraction.10,14 Muscle tissue has normally a low echogenicity. Its echogenicity is increased in the presence of fibrosis and by infiltration of fat tissue which may be caused by a number of neuromuscular disorders (see below).3-5,15 Sizes of human limb muscles, paraspinal muscles and even of cranial or facial muscles can be reliably measured with US.16-19 Individual muscle fibres which are striated, multinucleated cells, ranging from 10–100 µm in diameter, can be distinguished from each other with ultrahigh-frequency (>30 MHz) MUS,20 but not with standard MUS. Muscle fibres are organized in grouped bundles (fascicles) surrounded by fibroadipose septa called perimysium. Hyperechogenic perimysium can be distinguished from muscle fascicles with diameters of more than 2 mm. The highly echogenic epimysium allows distinguishing muscles from each other.10 Figs. 1A, 2A, 2E, and 3E show MUS images of normal muscle tissue surrounded by other tissues.
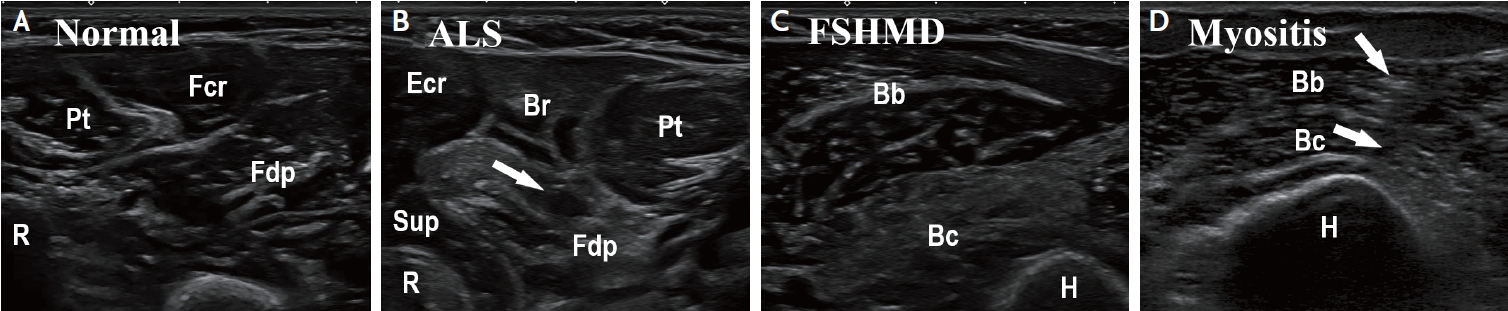
Muscle ultrasound findings in different neuromuscular disorders. (A) Normal forearm muscles with low, homogenous echogenicity. (B) Patient with amyotrophic lateral sclerosis. Note slightly increased echogenicity of Br, and markedly increased echogenicity of Fdp, with small areas of normal tissue inside (moth-eaten pattern, arrow). (C) Patient with facio-scapulo-humeral muscular dystrophy. Note homogenous, markedly increased echogenicity of the Bc. (D) patient with myositis. Note the patchy increase of echogenicity (arrows) in the Bb and Bc. Bb, biceps brachii; Bc, brachialis; Br, brachioradialis; Ecr, extensor carpi radialis; Fcr, flexor carpi radialis; Fdp, flexor digitorum profundus; H, humerus; Pt, pronator teres; R, radius; Sup, supinator.
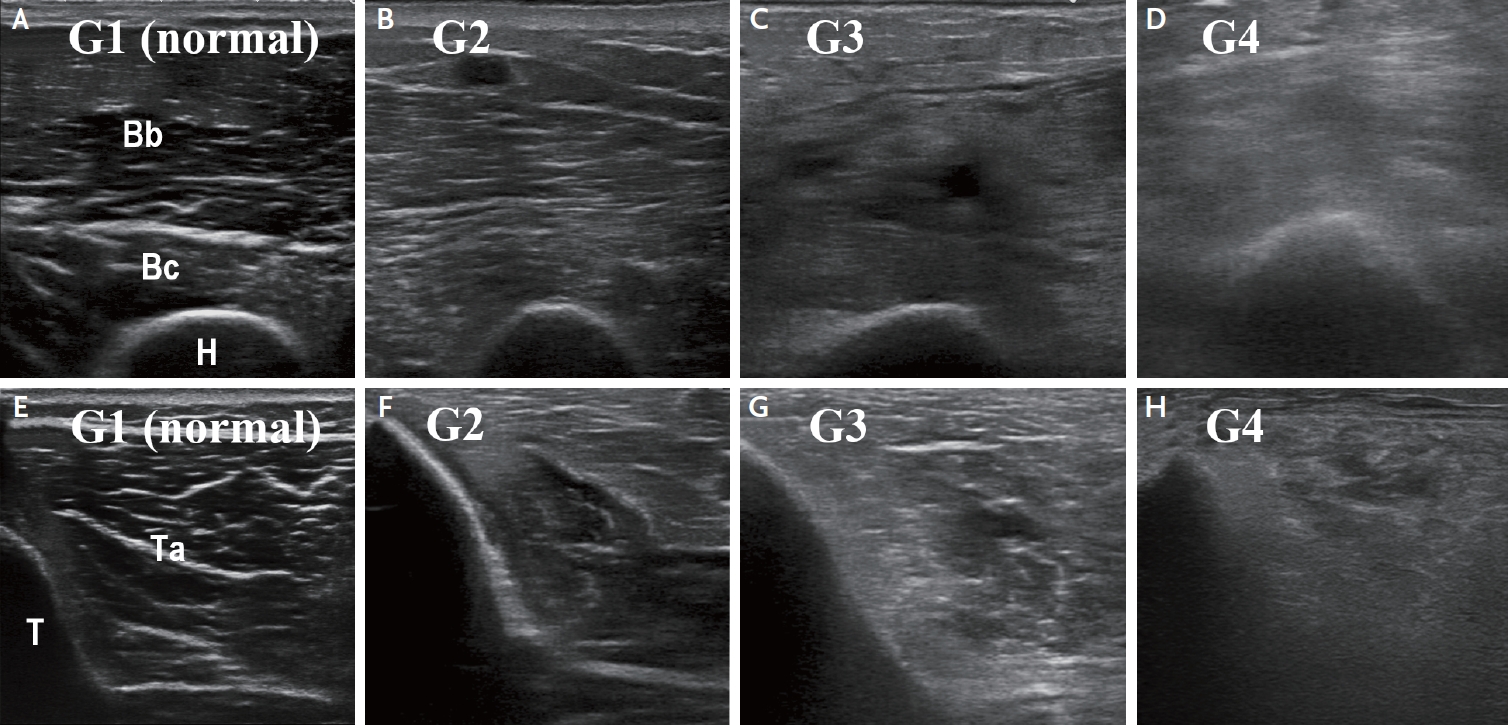
Qualitative (visual) grading of sonographic muscle changes on the Heckmatt scale. (A, E) Normal echo-intensity of the muscle with normal echo-intensity and full visualization of the adjacent bone cortex (grade 1). (B, F) Moderately increased echo-intensity of the muscle with normal echo-intensity and full visualization of the adjacent bone cortex (grade 2). (C, G) Markedly increased echo-intensity of the muscle with altered echo-signal and incomplete visualization of the adjacent bone cortex (grade 3). (D, H) Strongly increased echo-intensity of the muscle with complete loss of bone cortex echo-signal (grade 4). Bc, brachialis; Br, brachioradialis; H, humerus; T, tibia; Ta, tibialis anterior.
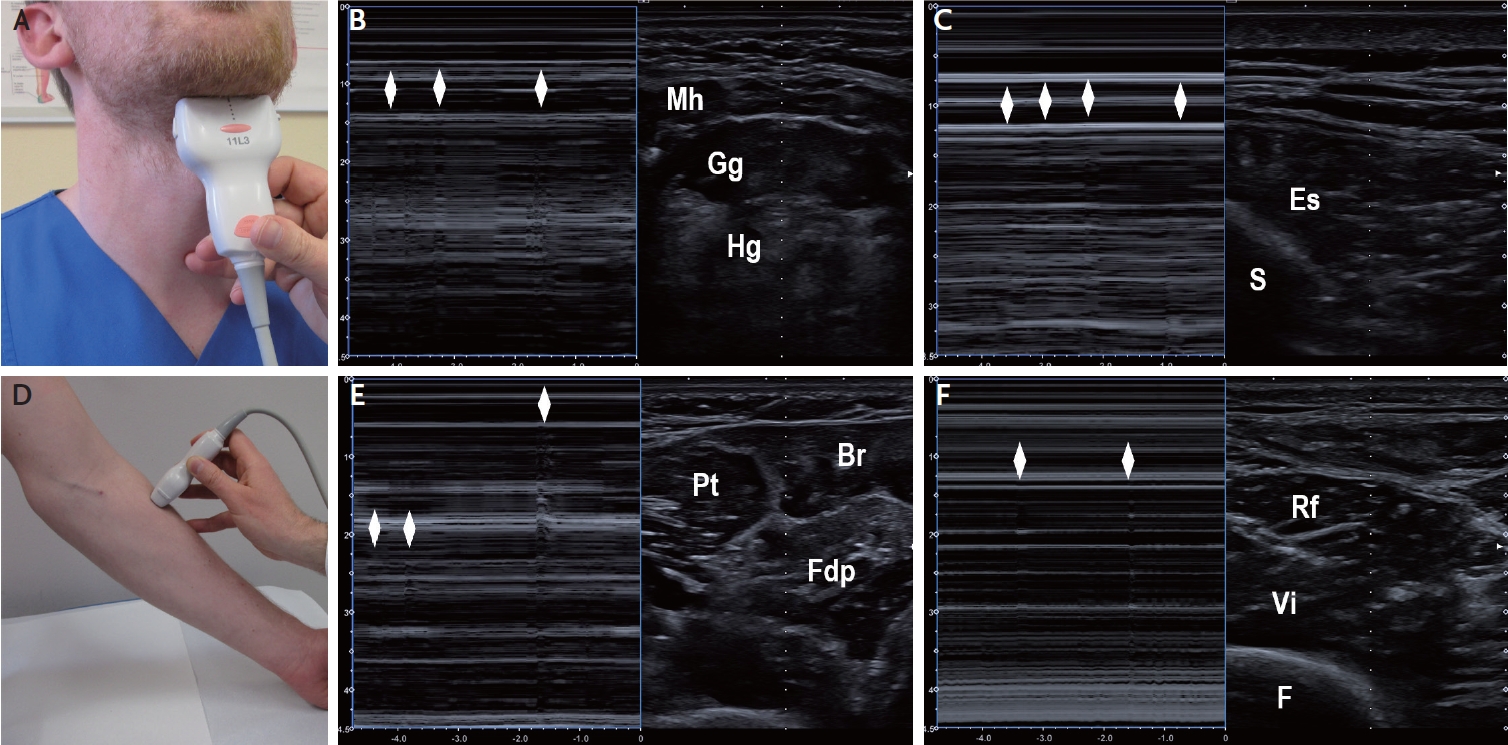
Detection of fasciculations on muscle ultrasound in patients with motor neuron diseases. (A) Positioning of the ultrasound transducer for visualization of tongue muscles displayed in (B). (B) Coronal B-mode sonogram (right panel) and corresponding M-mode sonogram (left panel) through tongue muscles. The rhombi denote fasciculations in the Gg. (C) Axial B-mode sonogram (right panel) and corresponding M-mode sonogram (left panel) through lumbar paraspinal muscles. The rhombi denote fasciculations in the Es. (D) Positioning of the ultrasound transducer for visualization of radial forearm muscles displayed in (E). (E) Axial B-mode sonogram (right panel) and corresponding M-mode sonogram (left panel) through proximal forearm muscles. The rhombi denote fasciculations in the Fdp and Br. (F) Axial B-mode sonogram (right panel) and corresponding M-mode sonogram (left panel) through anterior thigh muscles. The rhombi denote fasciculations in the Rf. Br, brachioradialis; Es, erector spinae; Gg, genioglossus; F, femur; Fdp, flexor digitorum profundus; Hg, hyoglossus; Mh, mylohyoid; Pt, pronator teres; Rf, rectus femoris; S, spinous process; Vi, vastus intermedius.
HEREDITARY AND INFLAMMATORY MYOPATHIES
Myopathies with degeneration of muscle fibers are characterized by changes of tissue composition in the affected muscles. Damaged muscle fibres are replaced with connective and fat tissue which results in increased muscle echo-intensity.21 Increased muscle echogenicity is most pronounced in chronic injury due to muscular dystrophy, myositis, or nerve injury without re-innervation (Fig. 1).22-24 Both, the echotexture pattern within an altered muscle and the pattern of involved muscles on MUS, can aid the differentiation of muscle disorders (Table 2). The increase of echo-intensity can be graded visually (qualitative MUS) or by use of a digitized image analysis software tool (quantitative MUS). For visual grading the most widely used scale is the Heckmatt scale (Table 3, Fig. 2).25 For digitized analysis MATLAB based software or the standard histogram function of Adobe Photoshop (Adobe systems Inc., San Jose, CA, USA) has been applied.26,27 Qualitative and quantitative assessment of muscle echo-intensity allowed for the detection of disease progression in Duchenne and facioscapulohumeral muscular dystrophy.26-28 Qualitative and quantitative MUS detected early changes of affected muscles in children with Duchenne muscular dystrophy prior to clinical motor regression.28 Regarding inclusion body myositis (IBM), quantitative MUS (echo-intensity) of the flexor digitorum profundus muscle and the gastrocnemius muscle discriminated well between IBM patients and healthy controls, unlike MUS measures of muscle thickness.29 Beside echo-intensity and muscle thickness, being assessed on B-mode images, advanced MUS using shear wave elastography has been reported to improve the detection of myopathies such as polymyositis and dermatomyositis.30 MUS can help determine the optimal muscle biopsy site (e.g., by placing a skin mark prior to biopsy), or can be used for real-time visualization and guidance of needle biopsy.23
INTENSIVE CARE UNIT ACQUIRED WEAKNESS
Critically ill patients commonly suffer from extensive skeletal muscle atrophy and a generalized reduction of muscle strength, a condition termed intensive care unit acquired weakness (ICUAW).31 ICUAW is triggered by critical illness, immobilization and prolonged mechanical ventilation. Typically the upper and lower extremities present with flaccid, symmetrical paresis while facial muscles and cranial nerves are spared. The pathophysiological correlate comprises critical illness myopathy and critical illness polyneuropathy, with both components present in 30–50% of patients, and pure myopathy in the majority of the other patients, while pure neuropathy is rare.32 Since specific diagnostic (e.g., laboratory) markers are not established yet, the diagnosis is based mainly on clinical examinations. However, due to prolonged sedation, delirium and a cognitive impairment, clinical assessment is often limited, if not impossible in critically ill patients.33 Fortunately, an increasing number of studies demonstrated the value of MUS in the diagnosis of ICUAW.34-41 Many studies assessed echo-intensity with quantitative or qualitative MUS,34,35,37-39,42 others employed measures of muscle thickness or pennation angle.36,40,41 Increased echo-intensity of muscle tissue in ICUAW patients has been related to inflammation-induced myofiber necrosis which occurred in about half of patients within a week of ventilation on ICU.43 Data of pilot studies suggest that MUS may be helpful for the outcome prediction of patients with ICUAW.36,39 Qualitative MUS (Fig. 2) might possibly be more useful compared to quantitative MUS in diagnosing ICUAW.39,40,42 Assessment of echo-intensity of arm muscles may be more valid than MUS of leg muscles in discriminating patients who develop ICUAW from those who do not.38,39 More recently, also shear wave elastography (SWE), superb microvascular imaging (SMI), and contrast-enhanced ultrasound (CEUS) were reported to add diagnostic value in ICUAW.44 If echo-intensity is used as a diagnostic marker of ICUAW, based on the current evidence the sum score of Heckmatt grades of the following muscles (each assessed bilaterally) should be calculated: biceps brachii, brachioradialis, forearm extensors, quadriceps femoris and anterior tibial muscle.34,35,38,39
PERIPHERAL NEUROPATHIES
In patients with chronic disease of peripheral nerves, typically atrophy of the denervated muscles occurs which can be measured with MUS.22,23,45 In classical distal-symmetric polyneuropathy, atrophy is most pronounced in distal muscles.45 The denervation causes degeneration of muscle fibres which are replaced with connective and fat tissue. This results in streaky, patchy increased echogenicity interspersed with areas of relatively spared muscle with normal, low echo-intensity (moth-eaten appearance; Fig. 1B).22,23
MOTOR NEURON DISEASES
Similar to the changes in peripheral neuropathies, the denervation of muscles occurring in amyotrophic lateral sclerosis (ALS) or spinal muscular atrophy (SMA) causes measurable atrophy, increase of echogenicity and moth-eaten patterns on MUS.22,23 Histologically, muscle fibrosis is the correlate of increased echo-intensity in ALS.46 In motor neuron diseases MUS especially of the diaphragm is of diagnostic and prognostic relevance since MUS allows assessment of diaphragm function and thickness.47-49 The early affection of the abductor pollicis brevis and first dorsal interosseous muscles in ALS, with relative sparing of the abductor digiti minimi, known as the split hand phenomenon, can well be documented as the split hand index (ratio) calculated from the echo-intensities of medial versus lateral hand muscles.50 A prominent diagnostic feature mainly of ALS, but also SMA, are fasciculations in the skeletal muscles affected by the progressive denervation.48,51 Fasciculations are assessed on B-mode MUS in combination with the M-mode (motion mode) function (Fig. 3). Is has been shown that MUS is superior to electromyography in detecting fasciculations in ALS.52 The highest frequencies of fasciculations have been reported for the following muscles: biceps brachii, extensor digitorum communis, abductor pollicis brevis, first dorsal interosseous, rectus femoris, vastus lateralis, and tibialis anterior, irrespective of the region of ALS onset.52-55 Scan time per muscle of ≥60 s increases the chance of detecting at least two fasciculations per affected muscle to more than 90%.54 To detect fasciculation in the tongue, MUS is usually performed with submandibular position of the probe (Fig. 3A), however, higher sensitivity has been reported with transoral MUS.56 The diagnosis of ALS can be established if fasciculations (regarded as signs of the 2nd motor neuron) are present in two different body regions, and if additionally signs of the 1st motor neuron are present.57 SMA (and ALS) can be discriminated from hereditary muscular disorder with high sensitivity and specificity, if fasciculations are present on MUS in at least two different muscles groups.51
ULTRASOUND GUIDANCE OF BOTULINUM NEUROTOXIN INJECTIONS
1. Technique
Optimal positioning of patients, physician, and MUS device should be secured when MUS-guided injection is intended.58 Accurate clinical investigation of the patients, skilful planning of BoNT dose and injection targets, and precise anatomic knowledge are prerequisites of treatment success.6,59-64 MUS is useful not only for targeting and documentation of the injection procedure but may also aid in the identification of hypertonic (spastic, dystonic) muscles, e.g., by comparing muscle thickness and echogenicity between affected and unaffected side,60,65 or by applying sono-elastography.66-68 It has been demonstrated that MUS guidance clearly improves the precision of needle placement, especially in small and deeply located muscles.69-72 In addition MUS avoids needle puncture-related nerve or vessel injury.6,72,73 BoNT injections are usually performed with 20-mm, 27-gauge (outer diameter: 0.40 mm) needles or 40-mm, 27-gauge needles.6 For profound muscles an 80-mm, 23-gauge (0.60 mm) needle or a 120-mm, 25-gauge (0.50 mm) spinal needle may be used. Disinfection of skin and ultrasound transducer should be performed with non-alcoholic agents since alcohol may harm the transducer surface. The target muscle is first identified on MUS, and the optimal probe position for injection guidance is explored. Then the injection needle is inserted adjacent to the ultrasound transducer. While insertion of the needle through regular ultrasound gel has been proposed to be safe, it may be preferable to remove the excess gel with a sterile swap before injection.6 In principle, there are two techniques of needle insertion with respect to MUS imaging plane: the in-plane technique (the complete needle path is displayed) and the off-plane technique (only the tip of needle in the target region is displayed). The “freehand” off-plane technique without external needle guide is sufficient for most applications of MUS-guided BoNT injection.6 In some cases, the in-plane technique (optionally performed using a needle guide holder and biopsy software) is preferable for the injection of deep neck muscles if delicate structures such as distorted vessels or nerves are near the planned needle path. Generally, the ultrasound transducer should be placed in a way that the target structure is displayed in the centre of the monitor screen. The inserted needle is visible with high echogenicity with its intensity depending on the needle size. The injected BoNT solution usually presents as weakly echogenic depot leading to a local volume increase of the injected muscle.6 MUS can also be combined with electromyography using an injection needle customized for simultaneous electromyography.74 The muscles most frequently injected with MUS-guidance in the author’s clinic are summarized in Table 4.
2. Cervical dystonia
Especially for small and/or deeply located neck muscles (levator scapulae, splenius capitis, semispinalis capitis, longissimus capitis, obliquus capitis inferior, scalenus anterior, scalenus medius, longus colli) MUS-guided injection is recommendable (Fig. 4).6,71-73,75,76 A number of cohort studies demonstrated the safety and efficacy of MUS-guided BoNT injection in cervical dystonia.6,61,75-77 Recently the results of the first prospective, partially-blinded study comparing MUS-guided versus non-guided BoNT injection in cervical dystonia proved a clear additional benefit of MUS guidance.78 This may potentially allow for the decrease of individually applied BoNT doses, thereby reducing a potential risk of secondary immunogenic treatment failure which seems to be higher with injection of neck muscles as compared to muscles in other body regions.79
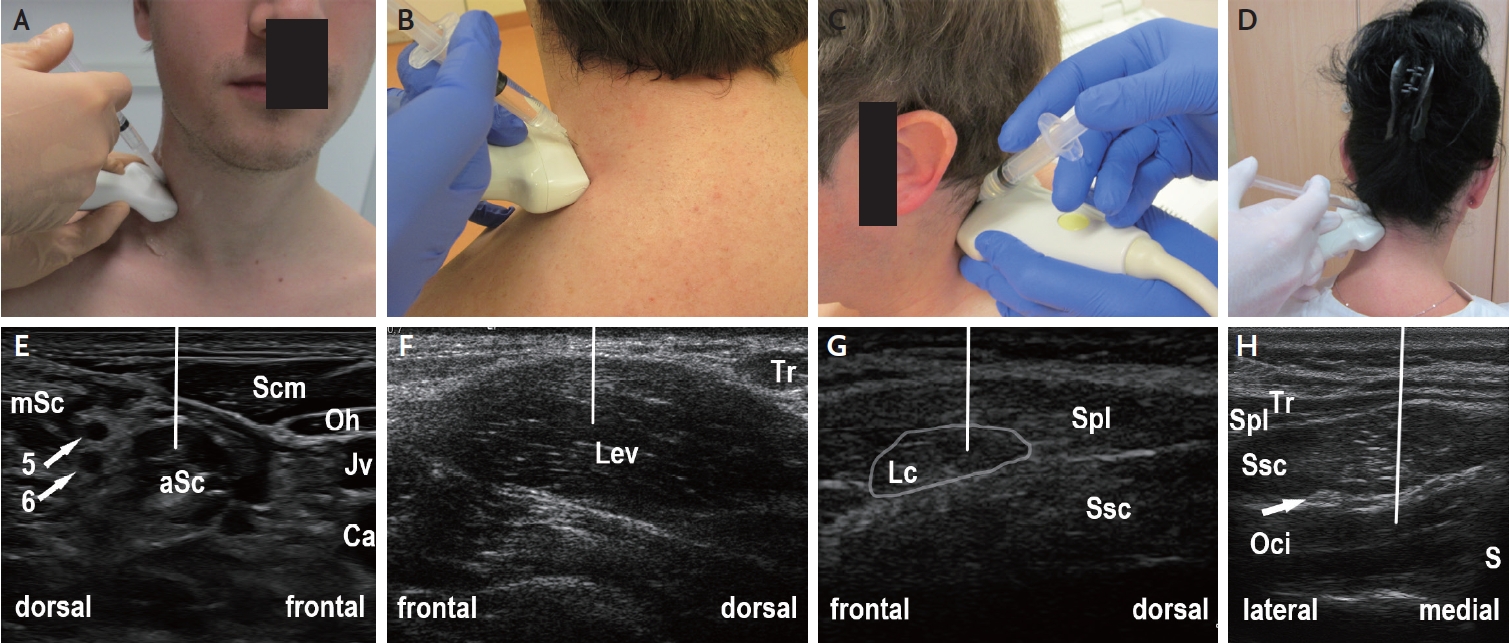
Muscle ultrasound of the lateral and dorsal neck region for guiding botulinum neurotoxin injection (off-plane injection technique). (A) Position of ultrasound transducer and syringe for guiding injection into the aSc. (B) Position of ultrasound transducer and syringe for guiding injection into the Lev. (C) Position of ultrasound transducer and syringe for guiding injection into the Lc. (D) Position of ultrasound transducer and syringe for guiding injection into the Oci. (E) Ultrasound image corresponding to (A) showing the lateral neck region. The aSc and mSc are discerned by visualizing the nerve roots of the brachial plexus (arrows, 5=5th cervical nerve root; 6=6th cervical nerve root). The line indicates the expected path of injection needle. (F) Ultrasound image corresponding to (B) showing the lateral neck region, with transducer position optimized for Lev injection. (G) Ultrasound image corresponding to (C) showing the lateral neck region, with transducer position optimized for Lc injection. (H) Ultrasound image corresponding to (D) showing the dorsal neck region, with transducer position optimized for Oci injection (arrow: greater occipital nerve). Note that the needle should be guided from medio-dorsal to slightly antero-ventral direction (indicated by the line) in order to avoid touching the vertebral artery. aSc, anterior scalene; Ca, common carotid artery; Jv, internal jugular vein; Lc, longissimus capitis; Lev, levator scapulae; mSc, middle scalene; Oci, obliquus capitis inferior; Oh, omohyoid; S, spinous process; Scm, sternocleidomastoid; Spl, splenius capitis; Ssc, semispinalis capitis; Tr, trapezius.
3. Task-related hand dystonia
Task-related dystonia (writer’s cramp, musician’s dystonia) involves forearm muscles that are dystonic only during specific activities. Therefore the therapeutic window of BoNT is small, and unintended paresis may occur. While there are no controlled studies assessing the use of MUS-guidance, there is expert consensus that a guidance technique (MUS or electrical stimulation) should be applied.6,80,81 A recent study found similar benefits with both guidance techniques, however favorable comfort of patients treated with MUS-guidance (Fig. 5).82 In many of our patients with writer’s cramp the BoNT dose could be reduced when switching from manual application to MUS-guidance.6
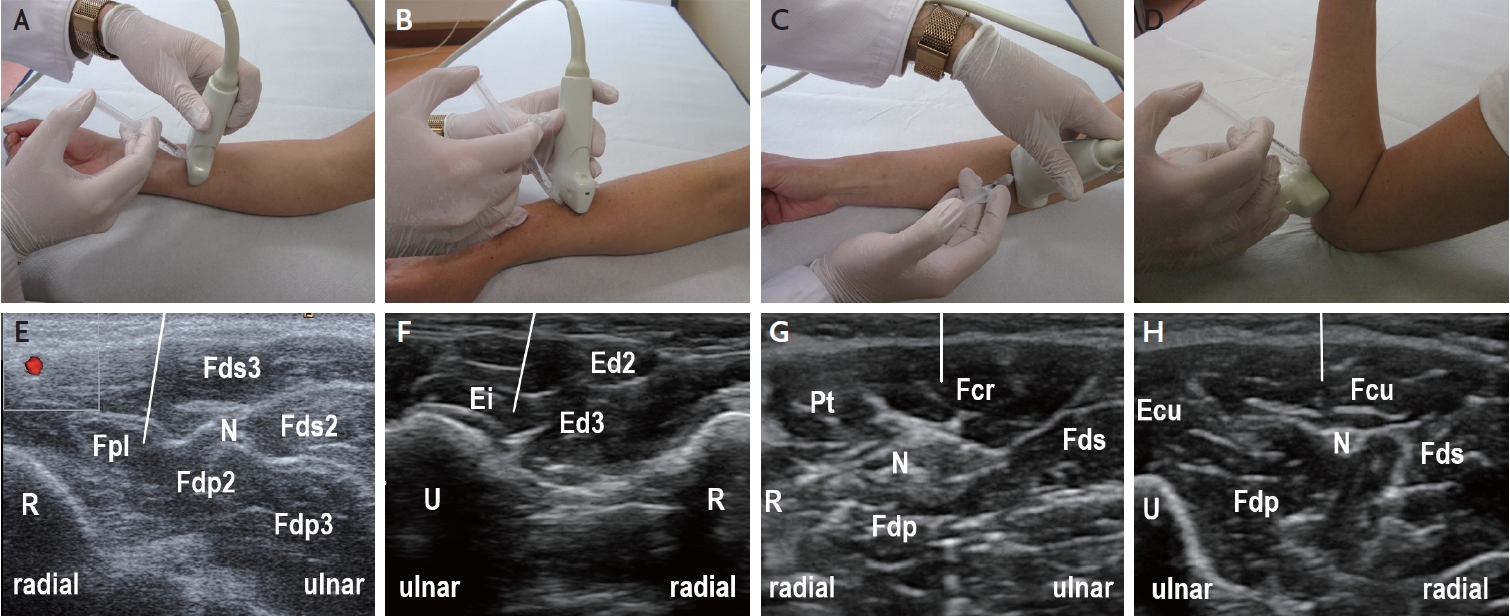
Muscle ultrasound of the forearm muscles for guiding botulinum neurotoxin injection (off-plane injection technique). (A) Position of ultrasound transducer and syringe for guiding injection into the Fpl. (B) Position of ultrasound transducer and syringe for guiding injection into the Ei. (C) Position of ultrasound transducer and syringe for guiding injection into the Fcr. (D) Position of ultrasound transducer and syringe for guiding injection into the Fcu. (E) Ultrasound image corresponding to (A) showing the distal forearm flexors. For guiding the injection into the Fpl, the radial artery (red) and the median nerve (N) should be clearly displayed. The line indicates the expected path of injection needle. (F) Ultrasound image corresponding to (B) showing the distal forearm extensors, with transducer position optimized for Ei injection. (G) Ultrasound image corresponding to (C) showing the proximal forearm flexors, with transducer position optimized for Fcr injection (N: median nerve). (H) Ultrasound image corresponding to (D) showing the proximal forearm flexors, with transducer position optimized for Fcu injection (N: ulnar nerve). Ecu, extensor carpi ulnaris; Ei, extensor indicis; Ed, extensor digitorum; Fcr, flexor carpi radialis; Fcu, flexor carpi ulnaris; Fds, flexor digitorum superficialis; Fdp, flexor digitorum profundus; Fpl, flexor pollicis longus; N, nerve; Pt, pronator teres; R, radius; U, ulna.
4. Cerebral palsy
Children and adolescents with cerebral palsy were among the first populations in whom MUS-guided injection techniques have been systematically applied.83 According to a European consensus statement,84 confirmed by subsequent systematic review,85 children with cerebral palsy should generally receive BoNT injections using EMG-guidance or MUS-guidance. Because of better tolerability, MUS-guidance is preferable in children. This applies also to adolescents and adults with cerebral palsy since chronic spasticity often led to muscular atrophy and joint deformation, requiring imaging-guided injection. MUS-guided BoNT injection provides a clear functional benefit to the patients.86 Findings of a recent sono-elastography study suggest that the treatment effect can be prolonged with extracorporeal shock wave therapy immediately following the BoNT injection.87
5. Poststroke spasticity
The use of intramuscular BoNT injection in poststroke spasticity is well established.88 Several small prospective randomised studies demonstrated superiority of MUS-guidance over the non-guided injection technique in patients with upper limb or lower limb spasticity.89-91 These findings were corroborated by observational studies.92-95 However, in another single-blinded, randomized controlled study the superiority of MUS-guidance could not be confirmed.96 In our experience MUS-guidance of BoNT injection in distinct muscles (Fig. 5, Table 4) may be favourable in patients with focal, or mild spasticity in whom precise targeting of a single muscle is required, and/or a functional benefit can be achieved.
6. Neurogenic thoracic outlet syndrome
Neurogenic thoracic outlet syndrome (nTOS) is a rare condition caused by compression of the brachial plexus at the thoracic outlet and presents with arm pain, paresthesias, and sometimes paresis of the hand. A minority of patients require decompressive surgery, however in many cases conservative treatment can be sufficient.97 Numerous case and cohort studies have shown a benefit of BoNT injection in the anterior scalene (Fig. 4A, 4E) and/or the pectoralis minor muscle.97 On the other hand, a double-blind, randomized, controlled trial did not find a significant improvement in pain or symptom reduction in nTOS patients.98 This study has been criticized because of EMG-guidance rather than imaging guidance, the very long symptom duration in the patients, and the inclusion of patients with mild symptoms at baseline.97 The results of a more recent study support the use of MUS-guided BoNT-injection.99
7. Piriformis syndrome
Piriformis syndrome is a neuromuscular condition caused by the entrapment of the sciatic nerve at the level of the piriformis muscle. It is diagnosed clinically by use of a set of specific tests, including the increase of pain during the FAIR (flexion, adduction, internal rotation) maneuver.100 This syndrome can well be treated with BoNT injection in the piriformis muscle. MUS-guided BoNT injection is more effective compared to MUS-guided injection of lidocaine or local ozone.100 Partial weakening of the piriformis muscle caused by the neurotoxin leads to decompression of the sciatic nerve which usually results in complete relief from pain. Usually this injection is done under computed tomography (CT-) guidance, which however necessitates the patients exposure to radiation and discomfort due to their prolonged positioning on the CT table. Moreover the sciatic nerve cannot always be visualized on CT. For MUS-guidance usually a 2–5 MHz curvilinear array transducer is used,100,101 however, in slender patients also a 6–13(–18) MHz linear array transducer is feasible (Fig. 6).100,102 This application requires higher intensity of antiseptic measures, the use of a long injection needle (e.g., a 120-mm, 25-gauge spinal needle), and should be performed by a physician who is well-experienced in conducting MUS-guided procedures.
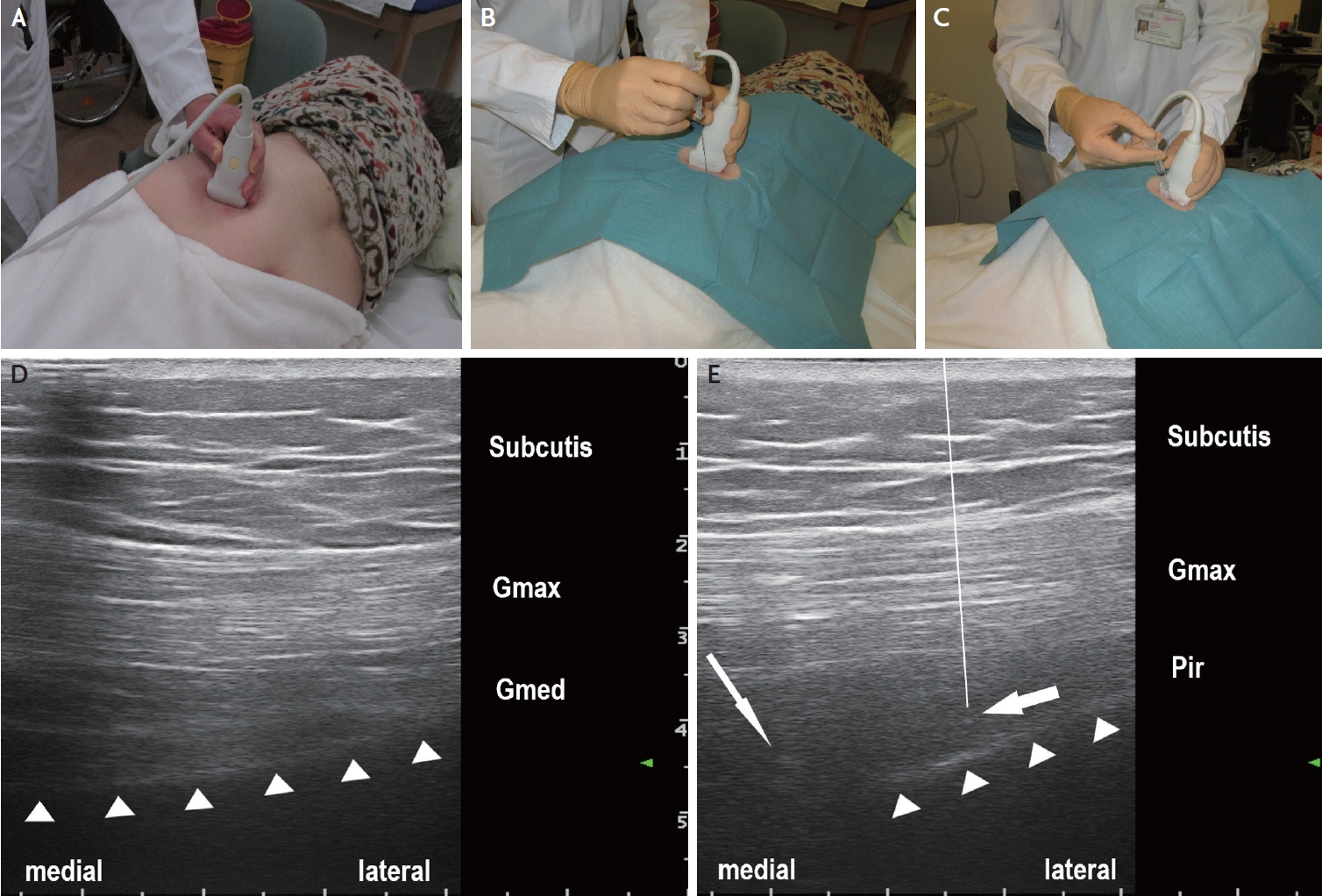
Ultrasound-guided botulinum neurotoxin injection into the piriformis muscle in a patient with severe, disabling piriformis syndrome. (A) Ultrasound transducer positioning for identification of the piriformis muscle. (B) Ultrasound-guided insertion of injection needle (here: 22-gauge/0.70×88 mm spinal needle) performed under antiseptic conditions. (C) Ultrasound-guided injection. (D) Semi-axial ultrasound imaging plane showing the gluteus maximus (Gmax) and the gluteus medius (Gmed) muscles and the iliac bone (triangles) cranial to the greater sciatic notch. (E) Semi-axial ultrasound imaging plane slightly caudal to the plane presented in (D). The piriformis muscle (Pir) is located with its lateral part between the gluteus maximus muscle (Gmax) and the iliac bone (triangles), and with its medial part between the gluteus maximus muscle and the greater sciatic notch (thin arrow: sciatic nerve). The thick arrow indicates the position of the needle tip in the piriformis muscle. The line indicates the path of needle which is not completely visible on the ultrasound image since an off-plane injection technique is used (with only the needle tip visualized in the target region).
CONCLUSION
MUS is increasingly used in neurology, neuropediatrics, neurosurgery and neuroradiology for the diagnosis of neuromuscular disorders. Especially, MUS is superior to other diagnostic tools in detecting fasciculations in motor neuron diseases and in revealing ICUAW. In addition MUS is an elegant tool for guiding needle biopsy and targeting intramuscular BoNT injection. Modern ultrasound technologies such as sono-elastography and high-definition ultrasound microvasculature imaging may allow for novel diagnostic and therapeutic uses.
Notes
Ethics Statement
The Institutional Review Board process and patient consents were not proceeded because of a review article.
Availability of Data and Material
The data supporting the findings of this review are available from the author upon request.
Sources of Funding
None.
Conflicts of Interest
The author has received speaker honoraria and travel reimbursement from Ipsen Pharma, Merz Pharma, and Allergan/Abbvie, and a research grant from Merz Pharma. He serves as Joint Editor-in-Chief of Ultraschall in der Medizin/European Journal of Ultrasound.
Acknowledgements
None.




